Skip to PhysioNet
Team
Publications
News
Research Summary
PhysioNet
(Physiology & Physiopathology of Brain Networks Team)
Skip to PhysioNet
Team
Publications
News
Research Summary
(Physiology & Physiopathology of Brain Networks Team)
PhysioNet is an international team of researchers from diverse backgrounds, pooling expertise in biology, physics, medicine, engineering, and mathematics.
Our work aims at understanding how neuronal network dynamics allow the exchange of information between brain areas in physiological and pathological conditions. We investigate how the information encoded in one neural structure is able to modulate the encoding in another area (and the other way around), how these interactions orchestrate perception, learning, and behavior, and how these processes are affected in epilepsy.
To do so, our primary approach is to perform high-density recordings of individual neurons simultaneously in multiple brain sites of freely behaving rats (healthy or epileptic) performing memory tests and then use data mining and analysis to study neural population dynamics.
Cognitive processes depend upon the activity of distributed networks in the brain. We use multiple silicon probes recordings in rodents sampling the network activities and the firing of a large number of neurons in the hippocampus, prefrontal cortex, entorhinal cortex and thalamic nucleus reuniens.
Our two main goals are:
(1) Understand the fundamental mechanisms underlying the communication between brain regions controlling memory processes by deciphering how cortico-thalamo-hippocampal networks exchange information to encode and consolidate spatial-related information.
(2) Understand how these physiological rules are modified in different pathological conditions, such as epilepsy.
We use data mining approaches to determine how the dynamics of hundreds of individual neurons and local network oscillations collected in animals performing memory-related tasks can support both memory function and dysfunction.
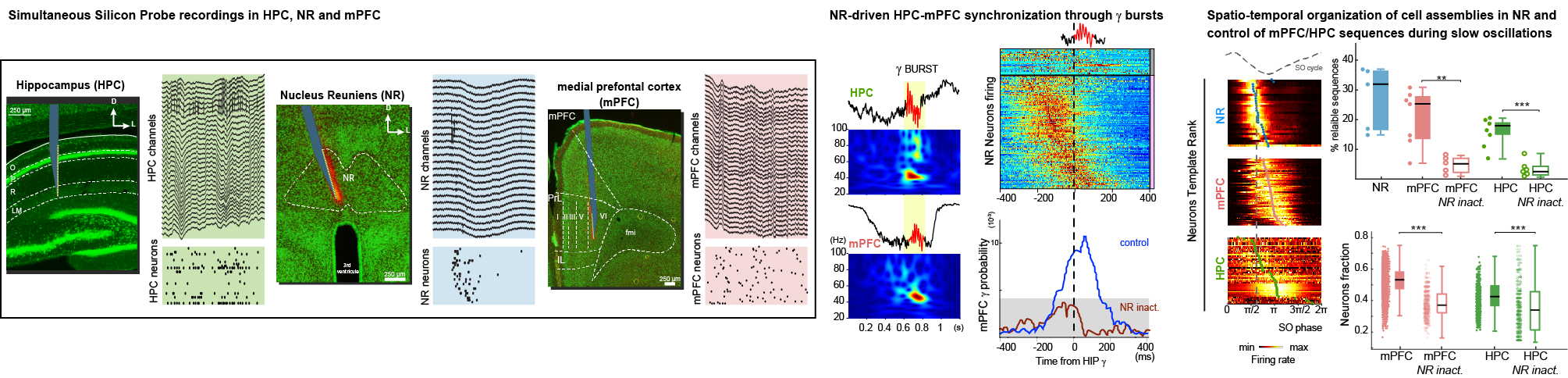
Click image to enlarge.

Mesial temporal lobe epilepsies (MTLEs) are refractory to pharmacological treatment. The most effective treatment for these epilepsies remains surgery, which is only effective in 60% of cases. This impasse is partly due to our partial understanding of the epileptogenic network characterizing these epilepsies. Electro-clinical studies provide evidence that these epilepsies are network diseases: The epileptogenic zone is a multi-structural network where the emergence of seizures requires not only the hyperactivity of neuronal populations but also the co-activation (synchronization of the activity) of the different limbic cortexes within the temporal lobe including the hippocampus, amygdala and entorhinal cortex. This led us to develop a research program to determine the structural and functional organization of the limbic neuronal networks, including sub cortical structures responsible of this paroxysmal activities and synchronization in the epilepsies. For this research, we combine innovative structural connectivity techniques using neurotropic viral vectors (rabies virus, AAV), neurochemical anatomy (immunohistochemistry, in situ hybridization, tissue clarification) and imaging (light, electron and confocal microscopy) with optogenetic and electrophysiological recording performed in rodent models of MTLE including several strains of transgenic mice as illustrated below.
Click image to enlarge.
Using mathematical and modeling approaches in close collaboration with the TNG team of V. Jirsa, we are studying the basic mechanisms of seizure genesis and propagation across species. We also investigate the mechanisms of vulnerability to epilepsy induced by stress as well as the co-morbidities such as depression, cognitive deficits (memory). We use a multi-disciplinary approach in healthy animals (rats and mice), experimental models of epilepsy (pilocarpine and kainite models) and Alzheimer (APPNL-G-F transgenic mice) in which we couple multisite recordings (in close collaboration with P. Quilichini group) and behavior.

Figures extracted from:
Ferraris et al (2018) J Neurosci 38(12):3026-3038. & Angulo-Garcia et al (2020) J Neurosci 40:8343-835.
Clawson et al (2023) J Neurosci 43(38: 6573-6587.
Billwiller et al (2020) Brain Struct Funct 225(9):2643-2668.
Ghestem et al (2023) J. Neural Eng. 20:046003.
Clawson W, Waked B, Madec T, Ghestem A, Quilichini P.P, Battaglia D, Bernard C. (2023). Perturbed Information Processing Complexity in Experimental Epilepsy. J Neurosci 43 (38) 6573-6587. doi: 10.1523/JNEUROSCI.0383-23.2023
Bernard, C, Frauscher, B, Gelinas, J, Timofeev, I. (2023) Sleep, oscillations, and epilepsy. Epilepsia 00: 1–10. doi: 10.1111/epi.17664
Ghestem A, Pompili MN, Dipper-Wawra M, Quilichini PP, Bernard C, Ferraris M (2023) Long-term near-continuous recording with Neuropixels probes in healthy and epileptic rats. J. Neural Eng. 20 046003. doi: 10.1088/1741-2552/ace218.
Doublet T, Ghestem A, Bernard C (2022) Deficit in observational learning in experimental epilepsy. Epilepsia 63 (12), e150-e155. doi: 10.1111/epi.17421
Rabuffo G, Sorrentino P, Bernard C, Jirsa V (2022) Spontaneous neuronal avalanches as a correlate of access consciousness. Frontiers in Psychology 13, 1008407. doi:
Brancati GE, Rawas C, Ghestem A, Bernard C, Ivanov AI. (2021) Spatio-temporal heterogeneity in hippocampal metabolism in control and epilepsy conditions. Proc Natl Acad Sci U S A. 118(11):e2013972118. doi: 10.1073/pnas.2013972118.
Ferraris M, Cassel JC, Pereira de Vasconcelos A, Stephan A, Quilichini PP. (2021) The nucleus reuniens, a thalamic relay for cortico-hippocampal interaction in recent and remote memory consolidation. Neurosci Biobehav Rev. 125:339-354. doi: 10.1016/j.neubiorev.2021.02.025.
Cassel JC, Ferraris M, Quilichini P, Cholvin T, Boch L, Stephan A, Pereira de Vasconcelos A. (2021) The reuniens and rhomboid nuclei of the thalamus: A crossroads for cognition-relevant information processing? Neurosci Biobehav Rev. 126:338-360. doi: 10.1016/j.neubiorev.2021.03.023.
Gomez-Castro F, Zappettini S, Pressey JC, Silva CG, Russeau M, Gervasi N, Figueiredo M, Montmasson C, Renner M, Canas P, Gonçalves FQ, Alçada-Morais S, Szabó E, Rodrigues RJ, Agostinho P, Tomé AR, Caillol G, Thoumine O, Nicol X, Leterrier C, Lujan R, Tyagarajan SK, Cunha RA, Esclapez M, Bernard C, Lévi S (201) Convergence of adenosine and GABA signaling for synapse stabilization during development. Science 374, eabk2055. doi: 10.1126/science.abk2055
Pedreschi N, Bernard C, Clawson W, Quilichini P, Barrat A, Battaglia D. (2020) Dynamic core-periphery structure of information sharing networks in entorhinal cortex and hippocampus. Network Neurosci. 4(3):946-975. doi: 10.1162/netn_a_00142.
Debski KJ, Ceglia N, Ghestem A, Ivanov AI, Brancati GE, Bröer S, Bot AM, Müller JA, Schoch S, Becker A, Löscher W, Guye M, Sassone-Corsi P, Lukasiuk K, Baldi P, Bernard C (2020). The circadian dynamics of the hippocampal transcriptome and proteome is altered in experimental temporal lobe epilepsy. Science advances, 6(41), eaat5979. doi: 10.1126/sciadv.aat5979
Angulo-Garcia D, Ferraris M, Ghestem A, Nallet-Khosrofian L, Bernard C, Quilichini PP (2020) Cell Assemblies in the Cortico-Hippocampal-Reuniens Network during Slow Oscillations. J Neurosci 40:8343-835. doi: 10.1523/JNEUROSCI.0571-20.2020
Vicente AF, Slézia A, Ghestem A, Bernard C, Quilichini PP. (2020) In Vivo Characterization of Neurophysiological Diversity in the Lateral Supramammillary Nucleus during Hippocampal Sharp-wave Ripples of Adult Rats. Neuroscience. 2020; 435:95-111. doi: 10.1016/j.neuroscience.2020.03.034.
Saggio ML, Crisp D, Scott JM, Karoly P, Kuhlmann L, Nakatani M, Murai T, Dümpelmann M, Schulze-Bonhage A, Ikeda A, Cook M, Gliske SV, Lin J, Bernard C, Jirsa V, Stacey WC (2020) A taxonomy of seizure dynamotypes. Elife 9:e55632. doi: 10.7554/eLife.55632.
Billwiller F, Castillo L, Elseedy H, Ivanov AI, Scapula J, Ghestem A, Carponcy J, Libourel PA, Bras H, Abdelmeguid NE, Krook-Magnuson E, Soltesz I, Bernard C, Luppi PH, Esclapez M (2020) GABA-glutamate supramammillary neurons control theta and gamma oscillations in the dentate gyrus during paradoxical (REM) sleep. Brain Struct Funct. 225(9):2643-2668. doi: 10.1007/s00429-020-02146-y.
Melozzi F, Bergmann E, Harris JA, Kahn I, Jirsa V, Bernard C (2019) Individual structural features constrain the mouse functional connectome. Proc Natl Acad Sci U S A. 116(52):26961-26969. doi: 10.1073/pnas.1906694116.
Clawson W, Vicente AF, Ferraris M, Bernard C, Battaglia D, Quilichini PP. (2019) Computing hubs in the hippocampus and cortex. Sci Adv 5(6): eaax4843, doi: 10.1126/sciadv.aax4843.
Manouze H, Ghestem A, Poillerat V, Bennis M, Ba-M'hamed S, Benoliel JJ, Becker C, Bernard C (2019) Effects of Single Cage Housing on Stress, Cognitive, and Seizure Parameters in the Rat and Mouse Pilocarpine Models of Epilepsy. eNeuro 6. doi: 10.1523/ENEURO.0179-18.2019
Baud MO, Ghestem A, Benoliel JJ, Becker C, Bernard C (2019) Endogenous multidien rhythm of epilepsy in rats. Exp Neurol 315:82-87. doi: 10.1016/j.expneurol.2019.02.006
Ferraris M, Ghestem A, Vicente AF, Nallet-Khosrofian L, Bernard C, Quilichini PP. (2018) The Nucleus Reuniens Controls Long-Range Hippocampo-Prefrontal Gamma Synchronization during Slow Oscillations. J Neurosci. 38(12):3026-3038. doi: 10.1523/JNEUROSCI.3058-17.2018.
Frigerio F, Flynn C, Han Y, Lyman K, Lugo JN, Ravizza T, Ghestem A, Pitsch J, Becker A, Anderson AE, Vezzani A, Chetkovich D, Bernard C. (2018) Neuroinflammation Alters Integrative Properties of Rat Hippocampal Pyramidal Cells. Mol Neurobiol. Sep;55(9):7500-7511. doi: 10.1007/s12035-018-0915-1.
Pietro BOZZO
Andrea NUTI
EMAIL: christophe.bernard@univ-amu.fr | pascale.quilichini@univ-amu.fr
PHONE: +33 4 91 29 98 06 | +33 4 91 32 42 31
Christophe Bernard is also Editor in Chief of the SfN journal eNeuro:
“If you wish to read my editorials on experimental bias, scientific rigour, reproducibility and statistics, please go to eNeuro Editorials”